Abstract
Introduction: The Sickle Cell World Assessment Survey (SWAY) was a cross-sectional survey to assess the global impact and treatment of sickle cell disease (SCD). Complications of SCD can lead to significant negative effects on patient (pt) quality of life. Recurrent vaso-occlusive crises (VOCs) are one of the most common SCD complications and can lead to poor quality of life and chronic organ damage. SCD manifestations can start as early as the first year of life. The implications of SCD on a child's life can be far reaching and may affect education, the global impact of which has not been well described. Here, we assess data from SWAY to better understand the impact of SCD on education among pediatric pts in the US vs other high-income countries (HIC) and low/middle-income countries (LMIC).
Methods: SWAY included individuals aged ≥6 years with a diagnosis of SCD. The survey was completed by proxy (parent/caregiver/guardian) for pts aged 6-11 years and could be optionally self-completed by pts aged ≥12 years. The survey consisted of 7 ratings-based (Likert scale) questions focused on education, where a score of 5, 6, or 7 indicated increasing levels of agreement. Pediatric pts were defined as those aged <18 years. Per the World Bank definition, HIC were defined as having a gross national income per capita of ≥US$12,536; LMIC represented all remaining countries. SWAY was not designed to assess treatment outcomes; all analyses are descriptive. Age groups were not matched, and pts were not followed up over time.
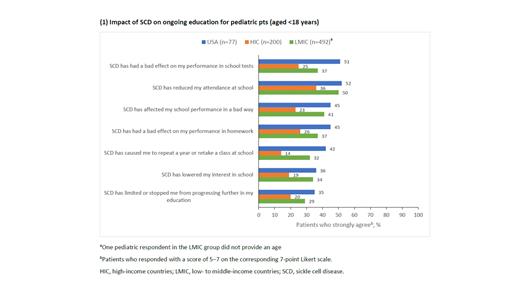
Results: Among the 769 pediatric pts participating in SWAY, there were 77 US respondents to the educational survey (mean age, 12 y), 200 HIC respondents (mean age, 13 y), and 492 LMIC respondents (mean age, 12 y, [one respondent did not provide an age]). Pediatric pts in all groups reported that SCD adversely impacted their education. Of the US respondents, 51%, 45%, and 52% agreed that SCD negatively impacted performance on school tests, overall performance at school, and school attendance, respectively. This was a higher rate of agreement for these statements than that reported by pediatric pts from other HIC (25%, 23%, 36%) and LMIC (37%, 41%, 50%). The US respondents also agreed that SCD negatively affected performance on homework (45%), caused them to repeat a year or class (42%), lowered interest in school (36%), and limited educational progression (35%). Again, this was a higher rate of agreement than that reported by pediatric pts from other HIC (26%, 14%, 19%, 20%) and LMIC (37%, 32%, 34%, 29%). Interestingly, the largest differences in reported school impact occurred between the US and HIC, where the US respondents showed nearly two-fold higher agreement for all statements except for reduced attendance. Conversely, there were only minor differences between respondents from the US and LMIC. Full results are presented in the Figure.
Conclusions: A higher proportion of pediatric pts in the US reported a negative impact of SCD on schooling compared with those in HIC and LMIC. These results were unexpected but align strongly with the emerging evidence that social determinants prevalent in the US lends itself away from the benefits of living in a resource-rich nation.
James: GBT: Honoraria; Novartis: Honoraria. Francis-Gibson: Global Alliance of SCD Organizations: Membership on an entity's Board of Directors or advisory committees; Sickle Cell Disease Association of America: Current Employment; Alliance for Regenerative Medicine Foundation for Cell and Gene Medicine: Membership on an entity's Board of Directors or advisory committees; ASH: Membership on an entity's Board of Directors or advisory committees; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees. Minniti: GBT: Consultancy, Research Funding; Novartis: Consultancy, Honoraria; NovoNordisk: Consultancy, Honoraria; Roche: Consultancy, Honoraria. Paulose: Novartis Pharmaceuticals Corporation: Current Employment. Bailey: Novartis Pharmaceuticals: Other: I am an employee of Adelphi Real World, which received payment from Novartis Pharmaceuticals for this research. Rajkovic-Hooley: Novartis Pharmaceuticals: Other: I am an employee of Adelphi Real World, which received payment from Novartis Pharmaceuticals for this research. Osunkwo: Forma Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Health and Services Administration: Research Funding; Patient Centered Outcomes Research Instituted: Research Funding; Micella Biopharma: Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Chiesi: Consultancy; Emmaus: Consultancy; Cyclerion: Consultancy; Acceleron: Consultancy; Global Blood Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Terumo: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal